Although treatment of RRMM is evolving, an unmet need remains—especially for triple class–exposed* patients.1-3
INDICATION
ABECMA (idecabtagene vicleucel) is a B-cell maturation antigen (BCMA)-directed genetically modified autologous T cell immunotherapy indicated for the treatment of adult patients with relapsed or refractory multiple myeloma after two or more prior lines of therapy, including an immunomodulatory agent, a proteasome inhibitor, and an anti-CD38 monoclonal antibody.
This website is best viewed using the horizontal display on your tablet device.

This website is best viewed using the vertical display on your mobile device.
Outcomes Are Poor in Triple Class–Exposed* RRMM Patients1,2
Although advancements in the treatment of RRMM have transformed outcomes, deep and durable responses remain difficult to achieve after patients have received an IMiD® agent, a PI, and an anti-CD38 antibody.1-3
When patients relapse, their disease becomes increasingly nonresponsive to the 3 main classes of treatments,1,4 resulting in:
- Shorter remissions between treatments4,5
- Rising burden of continuous administrations6,7
- Decreased quality of life6,7
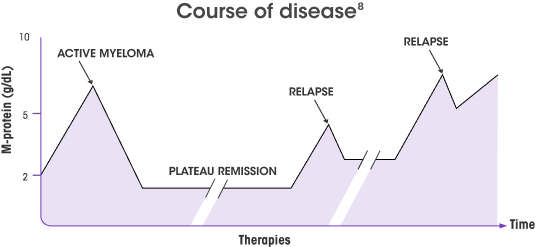
No standard of care exists for triple class–exposed* RRMM, and several studies have demonstrated poor outcomes in these patients4
Outcomes after
triple-class exposure*
26%-32%
ORR1,2,9,10
2%-3%
≥CR9,10
3-5 months
mPFS1,2,9-11
Novel mechanisms of action are needed in the management of RRMM1
After receiving multiple lines of therapy, patients’ T cell populations can become compromised, with fewer healthy T cells present. This can lead to12-14:
- Continued immune dysfunction
- Uncontrolled residual disease
Triple class–exposed* patients are in need of a novel treatment that:
- Can deliver deep responses with long durations of response5
- Alleviates the need for repeated administration15,16
- Increases quality of life6,7
Harnessing the power of the immune system early, while these immune cells are healthy, represents an important window of opportunity12
How Confident Are You in Your Treatment Approach for Triple-Class Exposed* Patients After 2L?
More patients with RRMM are becoming triple-class exposed* sooner1,2
- The increasing use of immunomodulatory agents, proteasome inhibitors, and anti-CD38 monoclonal antibodies has resulted in confident 1L and 2L treatment planning, but a faster path to triple-class exposure3,4*
Despite recent approvals and existing treatments, unmet need exists post
triple-class exposure2*


A growing number of patients with RRMM are triple-class exposed* after 2L.1,2
LocoMMotion is an ongoing prospective, noninterventional study in RRMM patients who have received ≥3 prior lines of therapy. Primary endpoint was ORR.5
Patients who have received an immunomodulatory agent, a PI, and an anti-CD38 monoclonal antibody.6
LocoMMotion is an ongoing, prospective, noninterventional study detailing the use of real-life current standard of care in the treatment of RRMM patients (n=248) who have received 3 or more prior lines of therapy or were double refractory to a PI and an immunomodulatory agent; received a PI, an immunomodulatory agent, and an anti-CD38 antibody; and had documented disease progression during or after their last line of therapy. 248 patients were enrolled between August 2, 2019 and October 26, 2020. The primary endpoint was ORR. Median follow-up was 11.01 months (range: 0.1-19.2). Patients had received a median of 4 prior lines of therapy (range: 2-13).5
1L=first-line; 2L=second-line; CR=complete response; mOS=median overall survival; mPFS=median progression-free survival; ORR=overall response rate; PI=proteasome inhibitor; RRMM=relapsed/refractory multiple myeloma.