INDICATION
ABECMA (idecabtagene vicleucel) is a B-cell maturation antigen (BCMA)-directed genetically modified autologous T cell immunotherapy indicated for the treatment of adult patients with relapsed or refractory multiple myeloma after two or more prior lines of therapy, including an immunomodulatory agent, a proteasome inhibitor, and an anti-CD38 monoclonal antibody.
This website is best viewed using the horizontal display on your tablet device.

This website is best viewed using the vertical display on your mobile device.
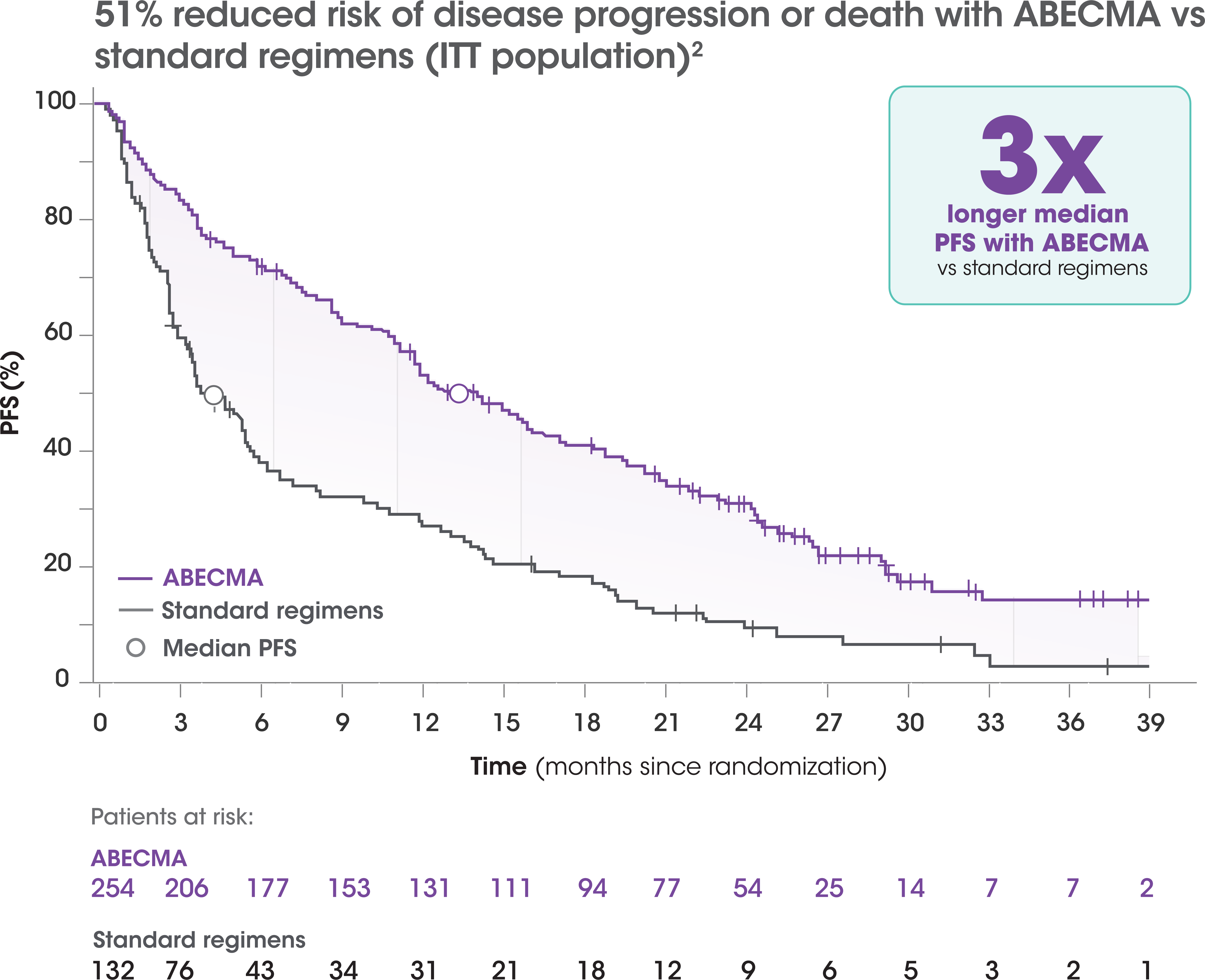
Triple-Class Exposed* Patients Experienced Superior Efficacy With ABECMA®1
Demonstrated superior PFS vs 5 standard regimens after 2L treatment2

NUMBER OF PATIENTS AT RISK
| Months | ABECMA | Standard regimens | ||
|---|---|---|---|---|
| 0 | 254 | 132 | ||
| 3 | 206 | 75 | ||
| 6 | 178 | 42 | ||
| 9 | 149 | 32 | ||
| 12 | 110 | 25 | ||
| 15 | 62 | 13 | ||
| 18 | 40 | 20 | ||
| 21 | 22 | 7 | ||
| 24 | 14 | 6 | ||
| 27 | 4 | 2 | ||
| 30 | 2 | 1 | ||
| 33 | 0 | 0 |
- Primary PFS analysis: Median PFS of 13.3 months (95% CI, 11.8-16.1) with ABECMA vs 4.4 months with standard regimens (95% CI, 3.4-5.9); HR=0.49 (95% CI, 0.38-0.64); P<0.0001 at 15.9 months of follow-up3†
- Final PFS analysis: Median PFS of 13.8 months (95% CI, 11.8-16.1) with ABECMA vs 4.4 months with standard regimens (95% CI, 3.4-5.8); HR=0.49 (95% CI, 0.38-0.63); at 30.9 months of follow-up2,4
- Patients in the control arm received the following therapies: DPd (33%), EPd (24%), Kd (22%), IRd (16%), DVd (6%)1
The efficacy analysis was performed on the ITT population of all randomized patients.5
Patients who have received an immunomodulatory agent, a PI, and an anti-CD38 monoclonal antibody.3
The primary efficacy analysis was performed on the ITT population of all randomized patients. For patients randomized to the ABECMA arm (n=254), this included 24 patients who were leukapheresed but did not receive ABECMA. For patients randomized to the standard regimens arm (n=132), this included 6 patients who did not receive treatment.5
2L=second-line; CI=confidence interval; DPd=daratumumab, pomalidomide, dexamethasone; DVd=daratumumab, bortezomib, dexamethasone; EPd=elotuzumab, pomalidomide, dexamethasone; HR=hazard ratio; IRd=ixazomib, lenalidomide, dexamethasone; ITT=intention to treat; Kd=carfilzomib, dexamethasone; PFS=progression-free survival.